Voxelotor Clinical Trial
We identified only two previous clinical trials evaluating voxelotor. Ad Are virtual clinical trials better.

Oxbryta Voxelotor For The Treatment Of Sickle Cell Disease
A Phase 3 Double-blind Randomized Placebo-controlled Multicenter Study of Voxelotor Administered Orally to Patients With Sickle Cell Disease.

Voxelotor clinical trial. N Engl J Med. Vichinsky E Hoppe CC Ataga Kl et al. One phase 12 ascending-dose open-label study of voxelotor and one previous report of the HOPE trial showing the efficacy and safety of voxelotor at 24 weeks.
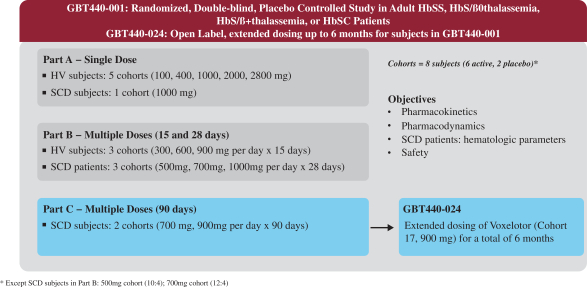
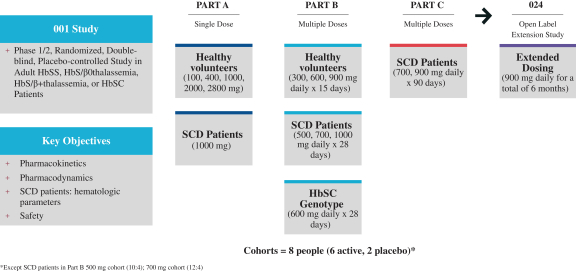
A phase 3 randomized trial of voxelotor in sickle cell disease. In the second part 24 participants aged 1217 received 900 mg of voxelotor a day. Participants were randomly assigned in a 111 ratio to receive a once-daily oral dose of 1500 mg of voxelotor 900 mg of voxelotor or placebo.
How feasible are they. In a multicenter phase 3 double-blind randomized placebo-controlled trial we compared the efficacy and safety of two dose levels of voxelotor 1500 mg and 900 mg administered orally once daily with placebo in persons with sickle cell disease. Oxbryta Full Prescribing Information.
Vichinsky E Hoppe CC Ataga Kl et al. N Engl J Med. A phase 3 randomized trial of voxelotor in sickle cell disease.
South San Francisco CA. The trial NCT02850406 was the first to assess voxelotor in pre-teens and teens. Pain was the most-frequent primary outcome in clinical studies completed before 2014 23.
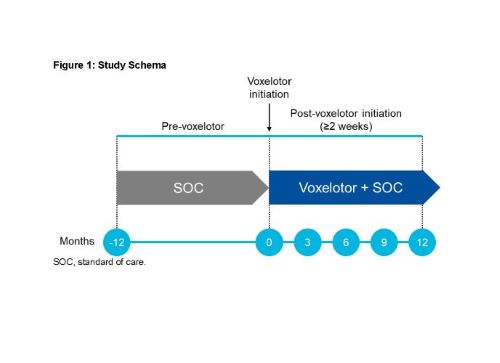
Separate from the clinical trials described a case series was published detailing the courses of seven patients with severe SCD treated with voxelotor on a compassionate use basis. Approximately 50 participants with sickle cell disease SCD aged 4 to 18 years will be enrolled at approximately 19 global clinical sites. At week 72 the adjusted mean change in haemoglobin concentration.
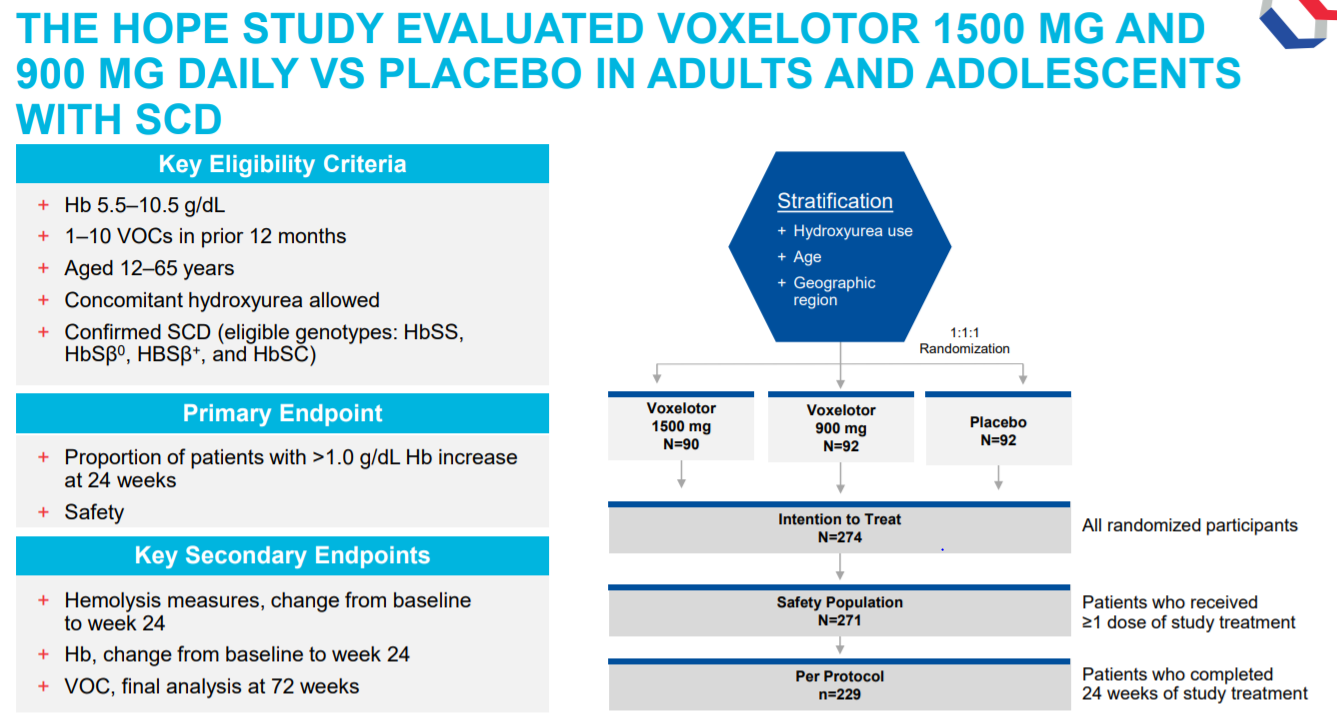
Five of the seven patients required frequent transfusions and one was refractory to transfusions and four patients had baseline oxygen saturations of. Phase 3 HOPE GBT440-031 is a randomised double-blind placebo-controlled multicentre clinical trial involving 274 patients with sickle cell disease. Between Dec 5 2016 and May 3 2018 449 patients were screened of whom 274 were randomly assigned to the voxelotor 1500 mg group n90 the voxelotor 900 mg group n92 or the placebo group n92.
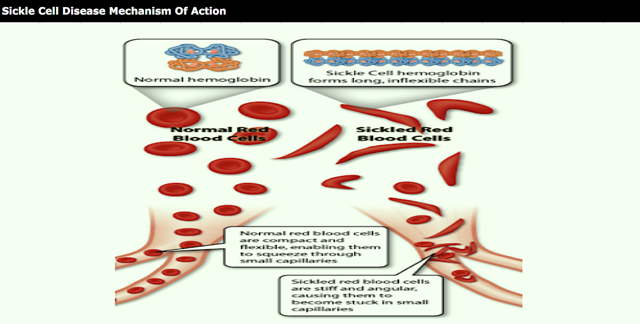
Voxelotor is an HbS polymerization inhibitor. After treatment improvement in hemoglobin was statistically significant in favor of 1500 mg voxelotor vs. Oxbryta Full Prescribing Information.
Earlier testing had been done only in adults. Download your free virtual clinical trials resource bundle. Researchers designed the study in two parts.
Find answers to these questions. Find answers to these questions. A Phase 3 Double-blind Randomized Placebo-controlled Multicenter Study of Voxelotor Administered Orally to Patients With Sickle Cell Disease.
N Engl J Med. Oxbryta Voxelotor Clinical Trials Update. A phase 3 randomized trial of voxelotor in sickle cell disease.
Oxbryta Voxelotor approval from the FDA comes from positive results of the Phase 3 HOPE Haemoglobin Oxygen Affinity Modulation to Inhibit HbS Polymerisation clinical study. The June 2021 Edition NTSs President Vice President Meet With Iraqi Government Officials To Discuss Thalassaemia Prevention National Programme. In a randomized placebo-controlled phase III clinical trial by Vichinsky et al.
39172 17 18 19. Ad Are virtual clinical trials better. The design called for evaluating a higher dose of the therapy in the first part of the trial.
Voxelotor demonstrated rapid sustained and clinically meaningful improvement in anemia sickling and clinical laboratory markers of hemolysis supporting the potential for voxelotor to serve as a disease-modifying therapy for SCD which is being further investigated in patients with SCD in the ongoing phase 3 HOPE study NCT03036813. This trial is registered with ClinicalTrialsgov NCT03036813. Open-label extension OLE study of voxelotor for pediatric participants with Sickle Cell Disease who have participated in voxelotor clinical trials.
Actual Study Start Date. N Engl J Med. Global Blood Therapeutics Inc.
VOCs an important SCD symptom and complication have been the principal focus of clinical trials 10 16 17. Download your free virtual clinical trials resource bundle. Global Blood Therapeutics Inc.
A phase 3 randomized trial of voxelotor in sickle cell disease. VOC a traditional endpoint for SCD. Actual Primary Completion Date.
2019 two doses of voxelotor 1500 mg N90 and 900 mg N92 were compared with placebo N92. The primary end point was the percentage of participants who had a hemoglobin response. How feasible are they.
Actual Study Completion Date. South San Francisco CA. 12-65 years old SCD patients were followed for 24 weeks.

Oxbryta Voxelotor For The Treatment Of Sickle Cell Disease
Real World Experience Of Patients With Sickle Cell Disease Treated Eha Library Andemariam B Jun 9 2021 324927
Global Blood Therapeutics Voxelotor Can Take The Stock Price To New All Time Highs Nasdaq Gbt Seeking Alpha

Voxelotor Clinical Trial Sickle Cell Society

Providing Hope To The Underserved Ppt Download

Fda Approves Oxbryta Voxelotor For The Treatment Of Sickle Cell Disease Cliniexpert

Providing Hope To The Underserved Ppt Download
Sickle Cell Disease Of 83 Of Teens Improves With Voxelotor Phase 2a Trial Shows

Efficacy And Safety Of Recently Approved Drugs For Sickle Cell Disease A Review Of Clinical Trials Experimental Hematology
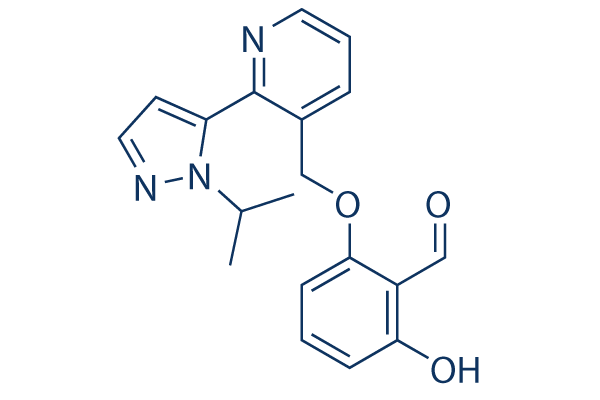
Voxelotor Gbt440 99 Hplc Selleck Others

Voxelotor In Adolescents And Adults With Sickle Cell Disease Hope Long Term Follow Up Results Of An International Randomised Double Blind Placebo Controlled Phase 3 Trial The Lancet Haematology

Providing Hope To The Underserved Ppt Download
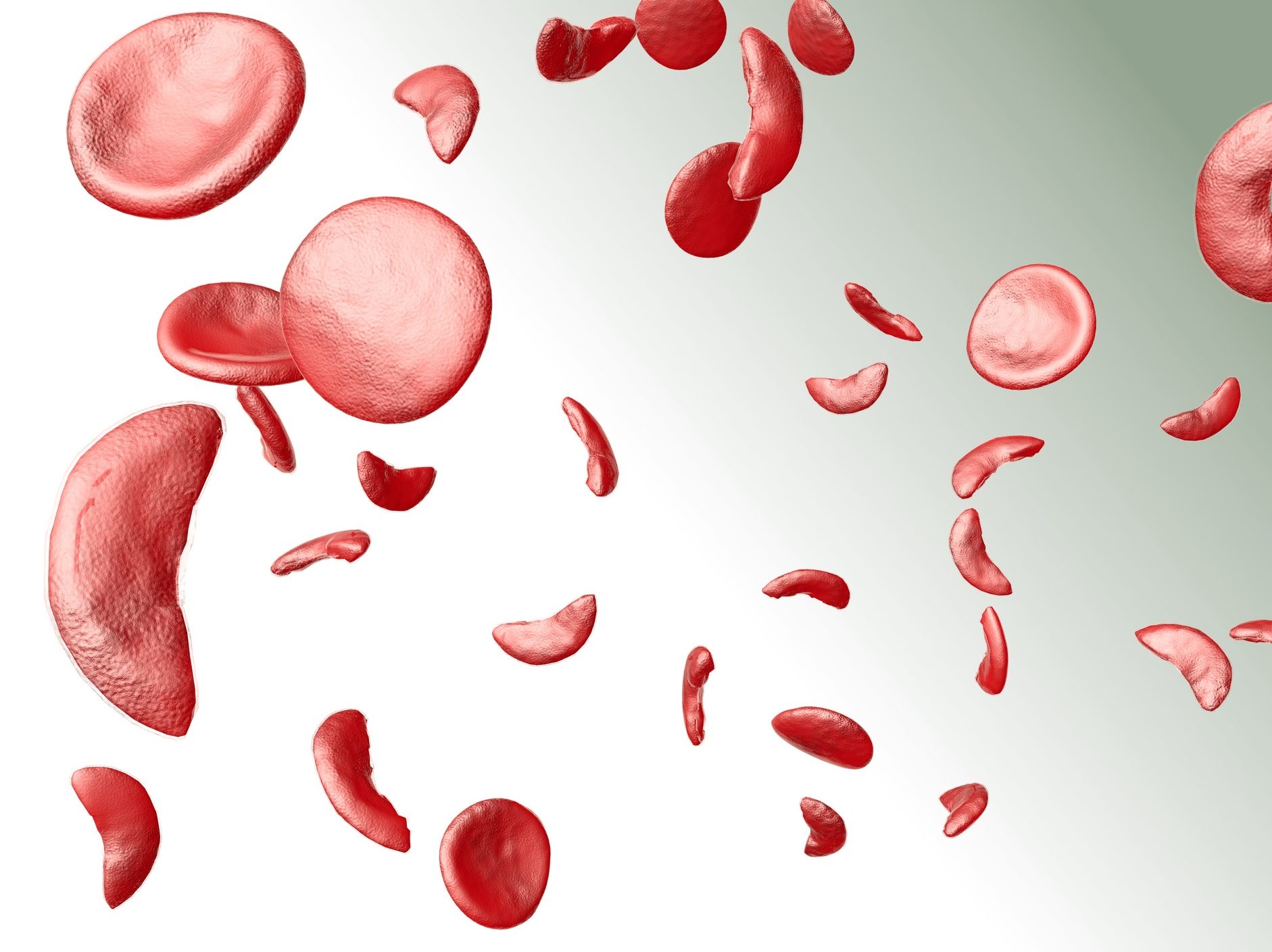
Voxelotor In Adolescents And Adults With Sickle Cell Disease Bjh

Gbt Announces Plans To Seek Regulatory Approval For Oxbryta Voxelotor To Treat Sickle Cell Patients In Europe Tif
-cas-1446321-46-5.png)

Post a Comment for "Voxelotor Clinical Trial"